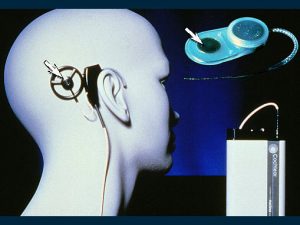
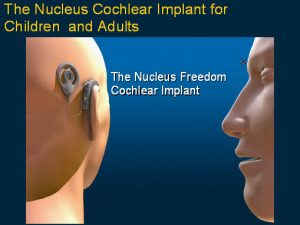
How the cochlear implant (bionic ear) functions
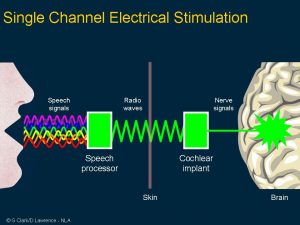
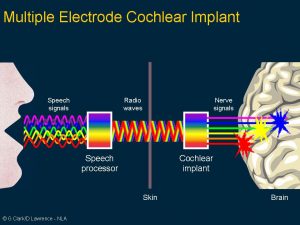
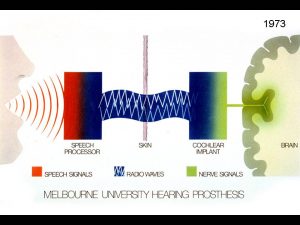
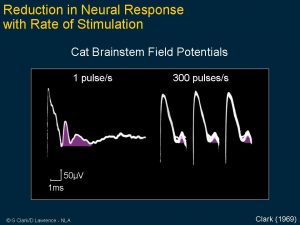 Clark’s first research aimed to see if electrical pulses could reproduce the time code for sound frequencies. His experimental work showed the brain stem nerve responses could follow electrical stimuli from 1 pulse per second and 300 pulses per second. Note that at high rates, the nerve responses disappear, indicating that a single channel implant would not be satisfactory for a cochlear implant.
Clark’s first research aimed to see if electrical pulses could reproduce the time code for sound frequencies. His experimental work showed the brain stem nerve responses could follow electrical stimuli from 1 pulse per second and 300 pulses per second. Note that at high rates, the nerve responses disappear, indicating that a single channel implant would not be satisfactory for a cochlear implant.
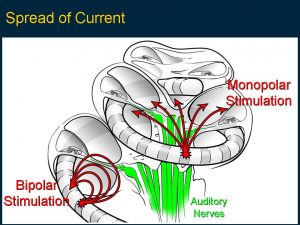
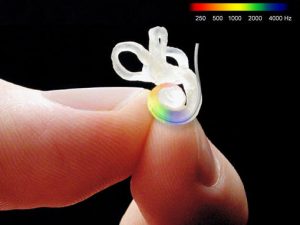 This image is a mould of the human cochlea showing how tiny it is. It has two and three quarter spirals around a central axis and the top loops for balance. The inner ear has three cavities which are about 1.5mm in diameter. There are up to 20,000 hair cells and 20,000 nerve fibres taking information to the brain. This was the challenge faced with a cochlear implant, how and what nerve to stimulate? The first evaluations on putting an electrode bundle into that spiral was that it would not pass up far enough to lie opposite the nerves transmitting speech frequencies. The answer came as shown in the next figure.
This image is a mould of the human cochlea showing how tiny it is. It has two and three quarter spirals around a central axis and the top loops for balance. The inner ear has three cavities which are about 1.5mm in diameter. There are up to 20,000 hair cells and 20,000 nerve fibres taking information to the brain. This was the challenge faced with a cochlear implant, how and what nerve to stimulate? The first evaluations on putting an electrode bundle into that spiral was that it would not pass up far enough to lie opposite the nerves transmitting speech frequencies. The answer came as shown in the next figure.
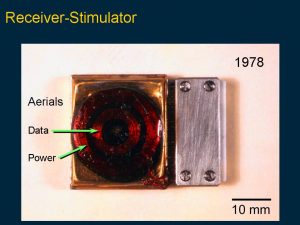
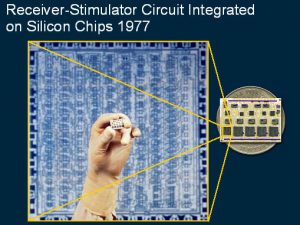 The biological and physiological studies were important in designing a prototype cochlear implant for tests on initial patients. This slide demonstrates the circuitry needed for the first prototype receiver-stimulator which had 10 silicon chips marked with the circuit design as shown in the enlarged circuit layout.
The biological and physiological studies were important in designing a prototype cochlear implant for tests on initial patients. This slide demonstrates the circuitry needed for the first prototype receiver-stimulator which had 10 silicon chips marked with the circuit design as shown in the enlarged circuit layout.
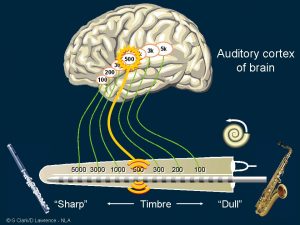 The interesting finding in the first patient was that stimulating a certain location in the inner ear was heard as a sharp or dull sound. It gave timbre rather than pitch. Timbre is the description for a musical sound that plays the same note but on a different instrument, so it’s the quality of the sound.
The interesting finding in the first patient was that stimulating a certain location in the inner ear was heard as a sharp or dull sound. It gave timbre rather than pitch. Timbre is the description for a musical sound that plays the same note but on a different instrument, so it’s the quality of the sound.
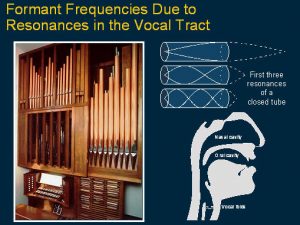
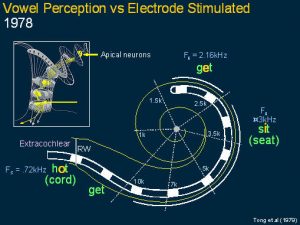 This slide shows a diagram of the cochlea and the first finding where the patient explained that stimulating different electrodes were heard not only as sharp and dull sounds but they were like vowels and the vowels varied according to the site of stimulation. It was noted that the frequency of site of stimulation corresponded to the frequency of a special signal in speech sounds called formants.
This slide shows a diagram of the cochlea and the first finding where the patient explained that stimulating different electrodes were heard not only as sharp and dull sounds but they were like vowels and the vowels varied according to the site of stimulation. It was noted that the frequency of site of stimulation corresponded to the frequency of a special signal in speech sounds called formants.
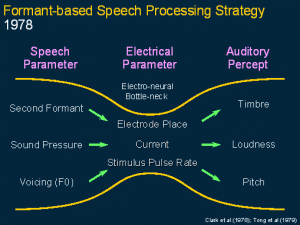 This shows the first strategy: the second formant frequency which is very important for intelligibility was coded for place of stimulation and perceived by the brain as timbre. Sound pressure was coded as current level and perceived as loudness and the voicing frequency was coded and stimulus pulse rate and perceived as pitch, and it was transmitted across each site of electrical stimulation.
This shows the first strategy: the second formant frequency which is very important for intelligibility was coded for place of stimulation and perceived by the brain as timbre. Sound pressure was coded as current level and perceived as loudness and the voicing frequency was coded and stimulus pulse rate and perceived as pitch, and it was transmitted across each site of electrical stimulation.
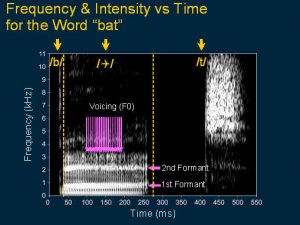 This illustrates that there are many features in speech signals that needed to be transmitted and coded. This speech spectrogram is for the word ‘bat’. Notice that the ‘b’ sound has a short burst of energy with different frequencies. The vowel ‘a’ has at least two strong bands of stimulation called the formants and the rate of stimulation relates to the voicing and the ‘t’ sound is a burst of high frequency energy.
This illustrates that there are many features in speech signals that needed to be transmitted and coded. This speech spectrogram is for the word ‘bat’. Notice that the ‘b’ sound has a short burst of energy with different frequencies. The vowel ‘a’ has at least two strong bands of stimulation called the formants and the rate of stimulation relates to the voicing and the ‘t’ sound is a burst of high frequency energy.
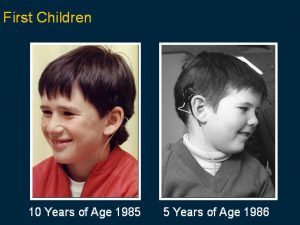 When it was shown to be effective and safe for adults, Clark and colleagues undertook the first implants on children from 1985 through to 1986. This was after extra special safety precautions were evaluated to see that it was safe in this age group as they have special issues, and it was also made smaller and did not have a connector but a magnet on it to hold the transmitting coil in place. This is an image of the first two young children operated on in 1985 and 1986.
When it was shown to be effective and safe for adults, Clark and colleagues undertook the first implants on children from 1985 through to 1986. This was after extra special safety precautions were evaluated to see that it was safe in this age group as they have special issues, and it was also made smaller and did not have a connector but a magnet on it to hold the transmitting coil in place. This is an image of the first two young children operated on in 1985 and 1986.
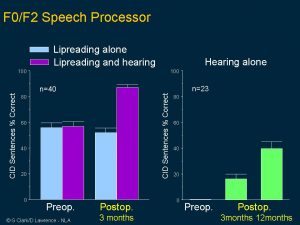
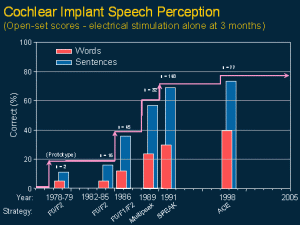
 Then in 1987 a world trial was commenced for the US FDA on children from two to 17 years of age. These are the best perceptual categories achieved for closed and open-set words both before and after electrical stimulation. Note the high proportion obtaining open or closed set speech understanding for electrical stimulation alone.
Then in 1987 a world trial was commenced for the US FDA on children from two to 17 years of age. These are the best perceptual categories achieved for closed and open-set words both before and after electrical stimulation. Note the high proportion obtaining open or closed set speech understanding for electrical stimulation alone.
In 1990 the FDA announced that the 22-channel cochlear implant was safe and effective for deaf children from two to 17 years of age in understanding speech both with and without lipreading. It was the first cochlear implant to be approved for deaf children by any world regulatory body.